PIPELINE
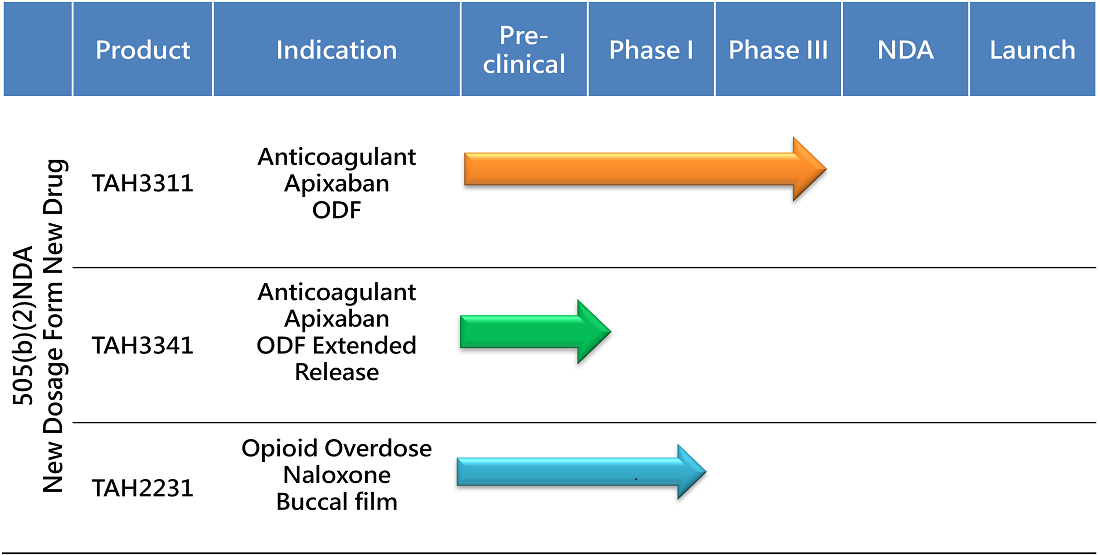
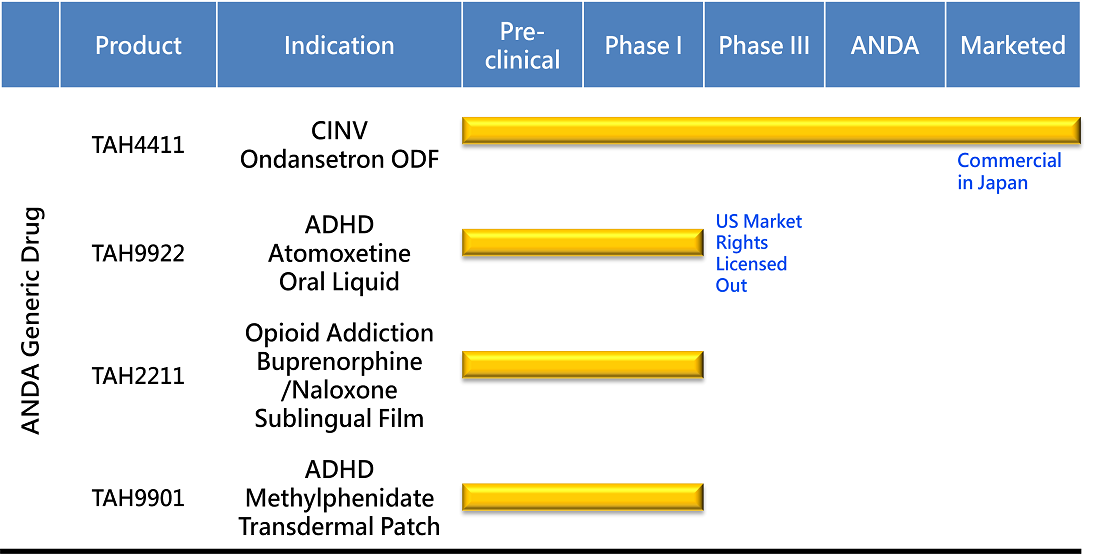
PRODUCT DEVELOPMENT
First Once Daily Anticoagulant Oral Film for Patients with Dysphagia
Along with TAH3311, TAHO is also developing an extended-release oral film that can treat stroke and systemic embolism caused by NVAF, a condition where the heart beats irregularly. Most of the existing treatments are either pills or injections, which are not suitable for many elderly patients who have trouble swallowing. Moreover, the current apixaban oral tablets need to be taken twice daily, making it hard to switch from other treatments that only require once-daily dosing.
TAHO’s extended oral film is the first of its kind that can deliver apixaban without the need for swallowing. Makes it easier and more convenient for both older and younger NVAF and PE patients. TAHO’s extended-release oral film aims to capture a large share of the global market for novel anticoagulants, which is worth more than $12 billion.

