PIPELINE
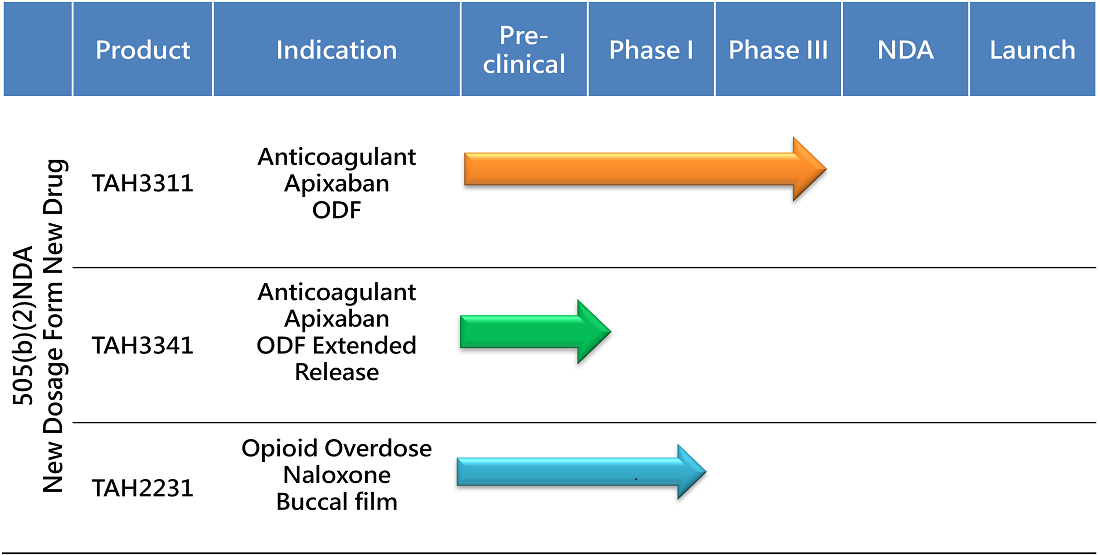
The 505(b)(2) NDA pathway streamlines drug development by leveragingexisting safety and efficacy data, reducing timelines, costs, and risks comparedto traditional methods. This approach accelerates market entry, offeringpatients safe, effective, and innovative treatments while addressing unmetneeds like improved dosage forms and tailored solutions for specificpopulations. TAHO leverages this pathway to deliver better therapies, bridgeinnovation with accessibility, and drive progress in healthcare.
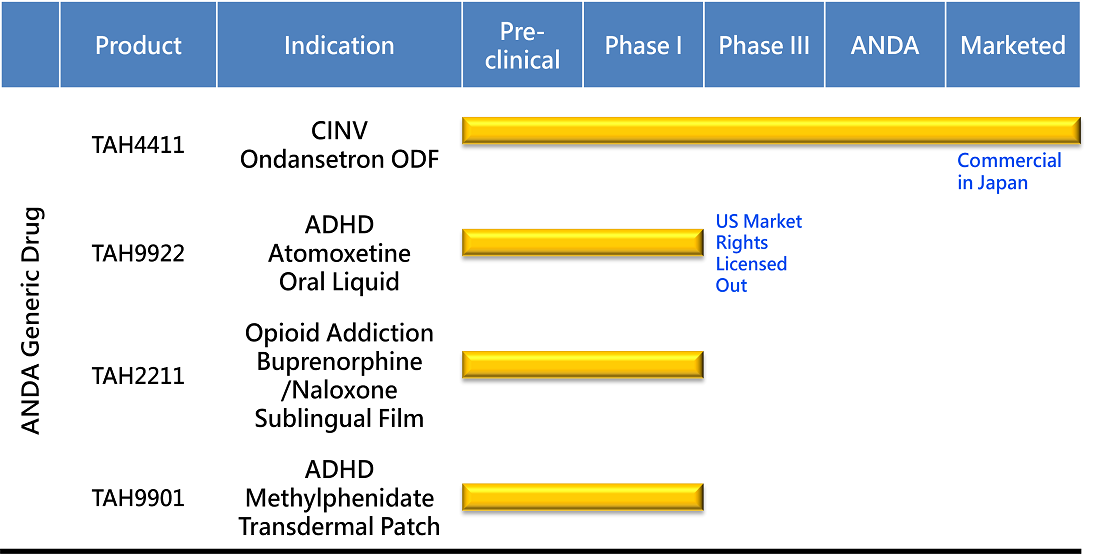
PRODUCT DEVELOPMENT
First Anticoagulant Oral film for Patients with Dysphagia
In reducing the risk of stroke and systemic embolism in nonvalvular atrial fibrillation (NVAF), all of the mainstream treatment options are oral tablets or injections. While most of the NVAF patients are elderly, many would have swallowing difficulty in taking pills.
To address this unmet need, TAHO is developing the first anticoagulant oral film that does not need swallowing. An easy and convenient medication for both elderly with NVAF or younger patients with pulmonary embolism (PE). Addressing an over $12 billion global novel anticoagulant market.

