PIPELINE
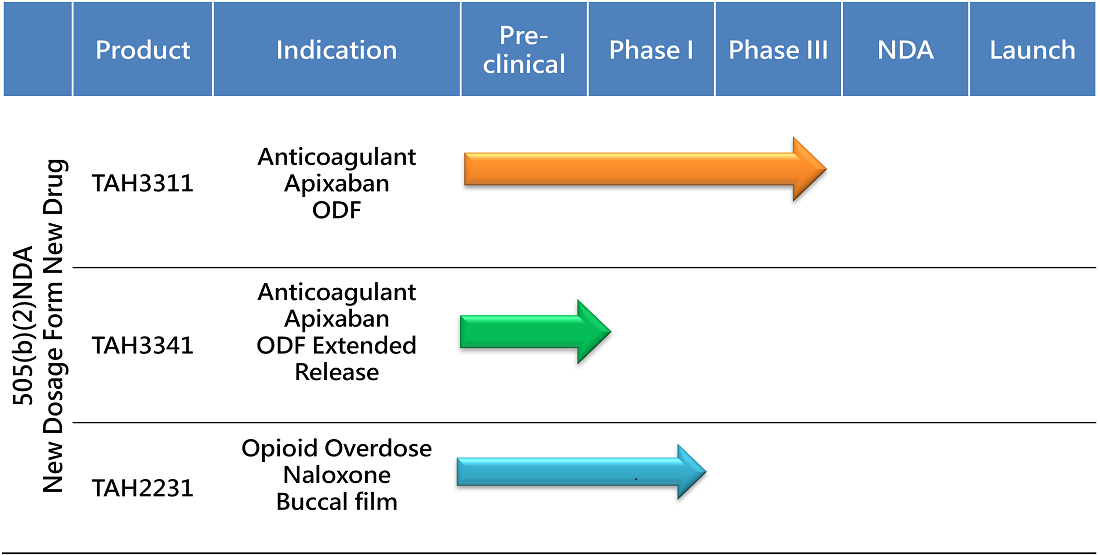
The 505(b)(2) NDA pathway streamlines drug development by leveragingexisting safety and efficacy data, reducing timelines, costs, and risks comparedto traditional methods. This approach accelerates market entry, offeringpatients safe, effective, and innovative treatments while addressing unmetneeds like improved dosage forms and tailored solutions for specificpopulations. TAHO leverages this pathway to deliver better therapies, bridgeinnovation with accessibility, and drive progress in healthcare.
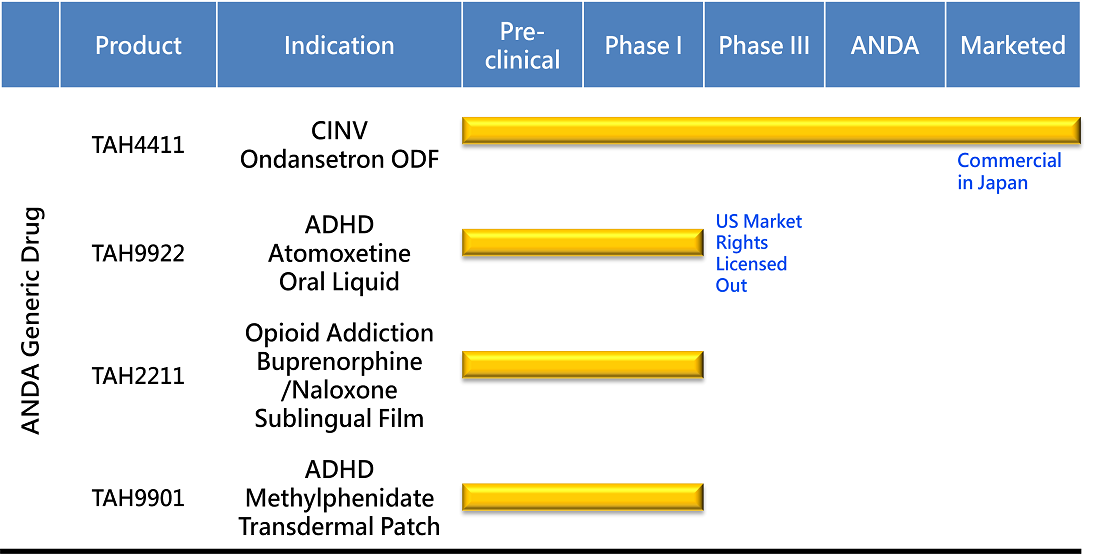
PRODUCT DEVELOPMENT
First Generic For Popular ADHD Treatment
Attention Deficit and Hyperactivity Disorder (ADHD) is usually first diagnosed in childhood and often lasts into adulthood. The current atomoxetine capsules and oral liquid are either difficult to swallow or have low palatability for pediatric patient.
Traversing this gap in pediatric need, TAHO's talented scientists have come up with a novel atomoxetine oral liquid with a pleasant mouthfeel in the form of a child-friendly oral liquid. This product is out-licensed to our partner as the first atomoxetine oral liquid in the US and is open for collaboration globally.

