PIPELINE
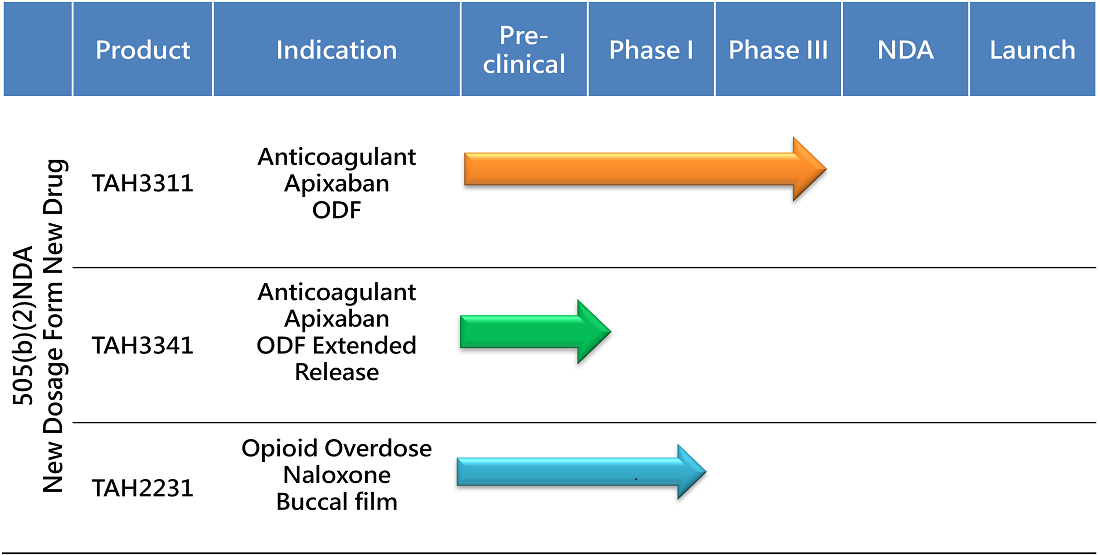
The 505(b)(2) NDA pathway streamlines drug development by leveragingexisting safety and efficacy data, reducing timelines, costs, and risks comparedto traditional methods. This approach accelerates market entry, offeringpatients safe, effective, and innovative treatments while addressing unmetneeds like improved dosage forms and tailored solutions for specificpopulations. TAHO leverages this pathway to deliver better therapies, bridgeinnovation with accessibility, and drive progress in healthcare.
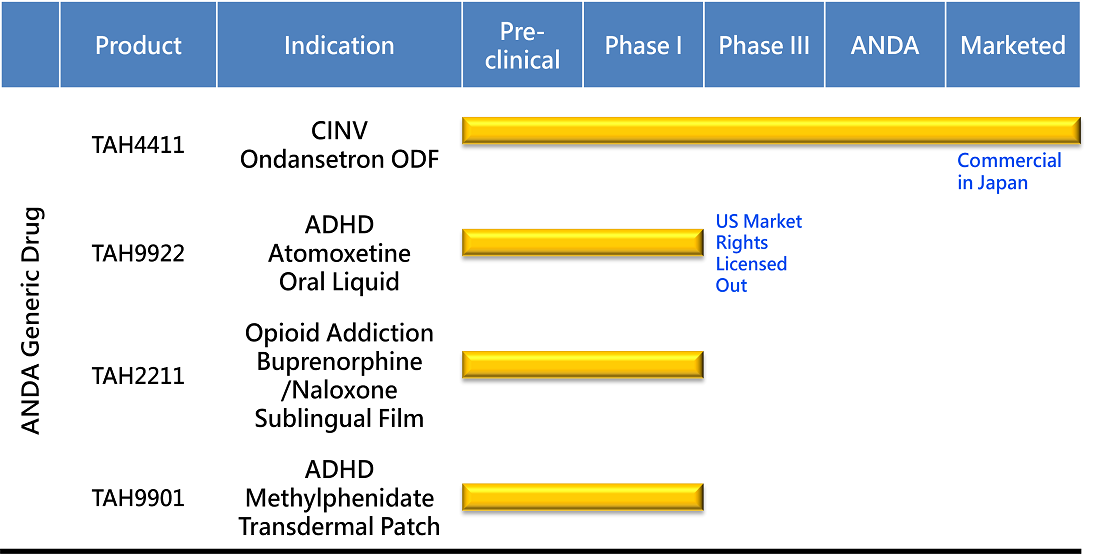
PRODUCT DEVELOPMENT
First Generic For Popular ADHD Treatment
Attention Deficit and Hyperactivity Disorder (ADHD) is usually first diagnosed in childhood and often lasts into adulthood. The current oral methylphenidate have unwanted side effects such as lower appetite and sleeping difficulty.
We are bridging this gap with transdermal delivery technology. Unlike oral medicine required a whole day to wear off it's side effects, the parent can easily terminate the medication by removing the patch from the skin. This once-daily patch is designed for 9 hours daily use, to avoid children taking medication on their own while keeping an optimal outcome at school.

