PIPELINE
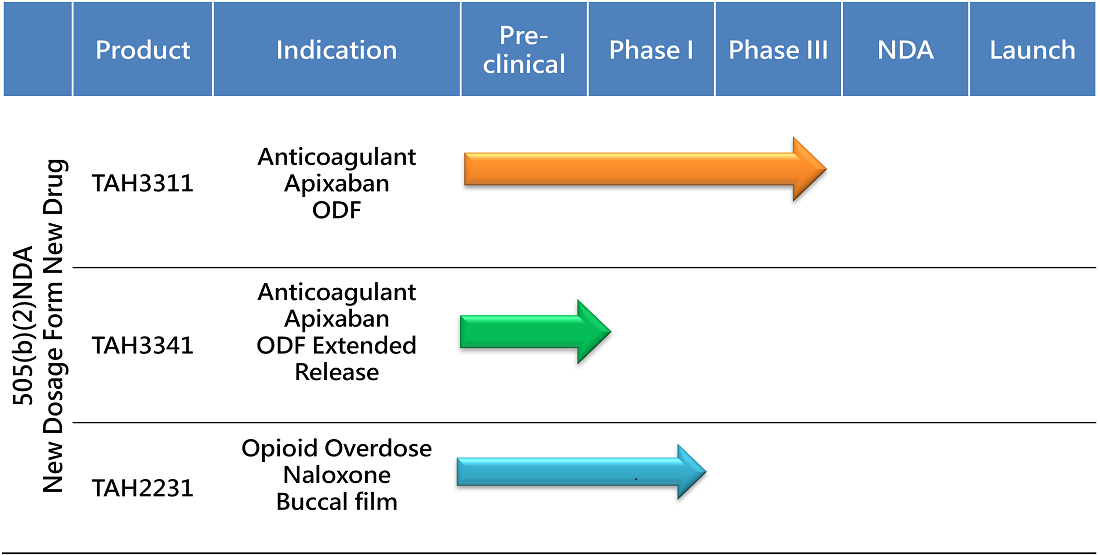
The 505(b)(2) NDA pathway streamlines drug development by leveragingexisting safety and efficacy data, reducing timelines, costs, and risks comparedto traditional methods. This approach accelerates market entry, offeringpatients safe, effective, and innovative treatments while addressing unmetneeds like improved dosage forms and tailored solutions for specificpopulations. TAHO leverages this pathway to deliver better therapies, bridgeinnovation with accessibility, and drive progress in healthcare.
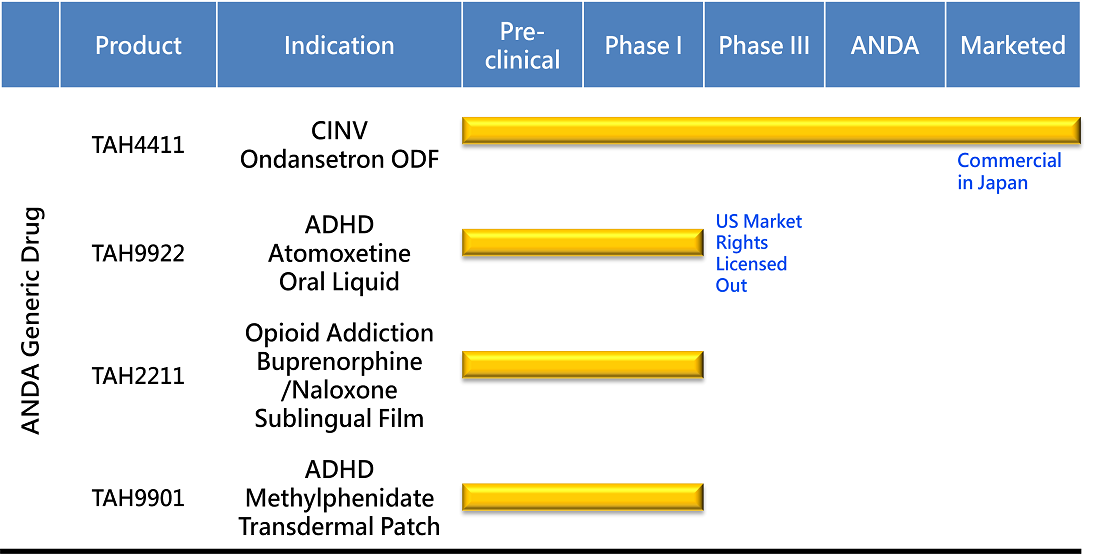
PRODUCT DEVELOPMENT
Commercial in Japan
TAHO's first approved product - A patient-friendly ODF formulation of a selective serotonin 5HT3 receptor antagonist for the treatment of chemotherapy-induced nausea and vomiting. Currently marketed in Japan.
This immediate release lingual film is designed for easy administration. As an anti-emetic treatment that does not require swallowing. It is an ideal at-home medication for the treatment of chemo therapy-induced nausea side-effect.

